Chemistry Uncovered: Your Guide to KS3 Chemistry Concepts
Chemistry is an exciting field that explores atoms, molecules, and chemical reactions. At Key Stage 3 level (KS3), chemistry builds upon concepts introduced during primary school study and provides the groundwork for more advanced study later. This guide will offer an overview of some critical areas covered by KS3 lessons.
The Particle Model
The particle model is one of the first concepts students learn in Key Stage 3 (KS3) Chemistry. This depicts the fundamental building blocks of matter: atoms and molecules. Students realise that all case is made up of tiny particles too small for us to see even with a microscope, explaining their properties and behaviours through how these tiny particles move or arrange themselves.
KS3 students use the particle model to visualise state changes. Solids contain tightly packed particles, while liquids and gases are free-moving. Heating or cooling materials cause their particles to speed up or slow down in response.
Elements, Compounds, and Mixtures
Understanding the differences between elements, compounds, and mixtures is fundamental to chemistry.
Elements
Are pure substances made up of only one type of atom? Examples are oxygen, iron, and helium. The periodic table organises all known elements based on their properties.
Compounds
Are pure substances of two or more elements chemically bonded in fixed proportions. For instance, water is a compound made of hydrogen and oxygen. No matter where water is found, it’s always H2O.
Mixtures
These are physical combinations of elements and compounds that are not chemically bonded. The composition of a mixture can vary. Examples include air, salad dressing, and granite.
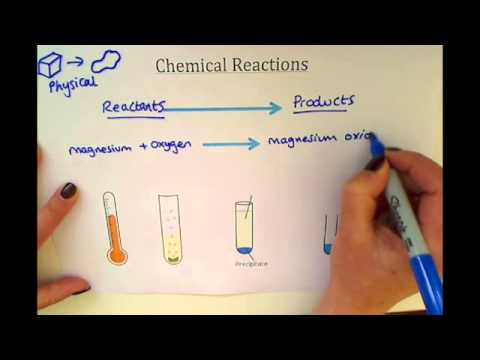
Chemical Reactions
Chemical reactions occur when substances change their chemical structure. KS3 students investigate different types of chemical reactions and how to represent them using chemical equations.
Some important types of chemical reactions covered in KS3 chemistry include:
- Combustion – A reaction between a fuel and oxygen that releases energy as heat and light. For example, the burning of methane gas: CH4 + 2O2 → CO2 + 2H2O
- Acid-base reactions – Acids and bases neutralise each other to form water and salt. For example, hydrochloric acid + sodium hydroxide → NaCl + H2O
- Oxidation – A reaction where a substance gains oxygen, loses hydrogen or loses electrons. The rusting of iron is an example of oxidation.
- Precipitation – Soluble ions in solution combine to form an insoluble solid called a precipitate. For example, silver nitrate + sodium chloride → AgCl precipitate.
Understanding how to interpret and balance chemical equations is an essential skill in chemistry.
Key Chemistry Concepts
Some other essential concepts covered in KS3 chemistry include:
Atoms and the Periodic Table
- Structure of atoms – neutrons, protons, electrons
- Periodic table patterns and trends
- Difference between elements, compounds, and mixtures
Bonding and Structures
- Ionic, covalent, and metallic bonding
- Properties based on bonding and structure
- Allotropes of carbon
Reactions
- Conservation of mass in chemical reactions
- Reactivity series of metals
- Acids and bases
Forces and Energy
- Particle model explanations of changes in state
- Endothermic and exothermic reactions
Earth and Atmosphere
- The composition of the Earth’s crust, atmosphere, and oceans
- The carbon cycle
- Greenhouse gases and climate change
Importance of KS3 Chemistry
KS3 Chemistry prepares students for continued science study by teaching fundamental concepts about matter’s structure, properties and interactions. Through hands-on lab work and explorations of real-world examples, it fosters critical thinking skills as well.
Understanding chemistry allows students to make sense of the world around them, from cooking and weather phenomena, down to their bodies, becoming less of an abstract concept when understood at a molecular level. KS3 chemistry lessons foster curiosity, improve scientific literacy, and open doors to more advanced engineering, healthcare and technology studies.
Conclusion
KS3 lessons provide students with a comprehensive introduction to the fundamentals of chemistry. Students gain an outstanding introduction through hands-on experience watching chemical reactions, analysing data, and critically considering matter and its components. Chemistry may initially seem complicated for students; however, through structured KS3 lessons, they gain domain-specific vocabulary, practical laboratory skills, and core knowledge needed to tackle more advanced concepts. Hands-on learning allows students to actively develop the skills of scientific observation, data interpretation and analytical reasoning.
Study of Key Stage Three Chemistry can unlock students’ potential as scientific thinkers, equipping them for success in high school chemistry classes and beyond. The organised curriculum gives students tools for comprehending our increasingly technological world on a molecular level, opening them up to become adept scientific thinkers ready to face new challenges head-on Students through dedicated study.